The term hydrocarbon includes everything from oil to coal. As the term is used today, it also includes everything with a carbon atom in the structure. This includes kerosene, gasoline, natural gas, and even some synthetic hydrocarbons like Bitunol®.
As we discuss these different hydrocarbons in this article, we will be focusing on oil and gas terms. However, there are several other terms that apply to both oil and gas such as condensate, gas condensate, liquid condensate, and product condensate.
While these terms are often used interchangeably, liquid Condensates are typically considered a different thing to froms of of of of of of of of of ofofofofofofofofofofofoaconditeprocessfromtoaconditeprocessfromtoaconditeprocessfromtoafrom.
Contents:
Molecular formula

The molecular formula for petroleum is a small circle with a line inside it. This molecule is known as an alkyl group group. It consists of an alphabetical group that can be chain, compound, or solid.
The alkyl group can be chain or compound. A chain refers to a series of oil or grease lines connected together to flow a product. A compound contains different oils or grease lines that do not communicate together, but are combined into one substance.
Petroleum is one of the original products discovered. After being introduced in the late 1600s and early 1700s, petroleum was scarce for a time. Then, some brilliant people developed ways to use it and distribute it. Today, it is one of the most popular products sold sold.
Carbon number (z)

The number of carbon atoms in a molecule is called its carbon number. All molecules have a different carbon number, making it possible to identify individual compounds.
The more carbon atoms in a molecule, the more powerful it is. This is why some drugs have such powerful effects!
Many molecules contain alcohols as their main ingredient. These alcohols are commonly called gasoline-like chemicals. The reason these chemicals are named gasoline-like is because they can easily be burned into oil for lamps, flatscreen televisions and computers’ monitors.
However, this process does not last forever, so when it is time to use it, you must find something else to do the job. This method is similar to how oil & gas companies name their fuels. You have your usual names, but this time with your product.
Hydrogen number (y)
The hydrogen number is a well-known number used to describe the composition of gasoline and diesel fumes. Many people are unfamiliar with the hydrogen number.
It is used to describe the smell of gas, and in fact, many motorists are unfamiliar with the term. When gas is present, it has a lower hydrogen number than diesel.
This refers to gasoline containing more carbon monoxide than diesel does. When carbon monoxide is present in a fuel, it inhibits the combustion process and causes engine damage. As car gas prices have risen in recent years, there has been a demand for more efficient fuels such as compressed natural gas (CNG).
Sum the zeros of the carbon numbers
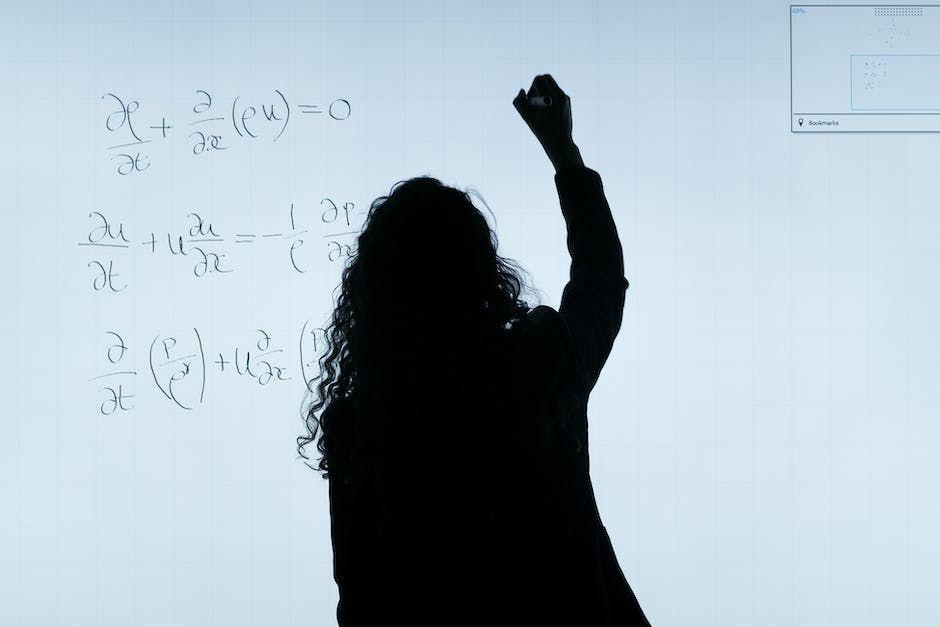
A carbon content of 10% means that 100 grams of the material contains 10 grams of carbon. Carbon is an element that can be found in many forms.
Many modern materials contain small amounts of carbon. Carbon is a common material component, making it possible to create many different products. For example, car paint and wood are two examples of materials that contain carbon.
Carbon is present in organic compounds such as pesticides and drugs, cooking oils and fats, energy sources such as drink powders and snacks, and in forensics materials. All of these examples may have a carbon content between 0% and 10%.
Sum the zeros of the hydrogen numbers

The total number of hydrogen atoms in a hydrocarbon is called the total hydrogen content (THC) of the petroleum or gas.
The total number of carbon atoms in a hydrocarbon is called the total carbon content (CCC). The two together make up a hydrocarbon.
A “n-alkyl”, or straight-chain alkyl, oil contains one to four ethynyl groups. An “n-butyl”, or butyl oil contains one to four pentynyl groups. A “m-butyl”, or butoxoethyl oil contains one to four hexynylethoxyethoxygroups.
As an example, ethylene glycol contains 1 ethinyl ester and 2 CCC CCCH HOC , for an average of 3 carbons perhydrogen and 4 CCC CCCH HOC .
Divide the sum of hydrogen numbers by the sum of carbon numbers

This equation can be applied to any substance, not just hydrocarbons. It can be used to determine the ratio of hydrogen to carbon in a molecule, and how much of each element is in a molecule.
The empirical formula of a substance is a reliable way to determine the ratio of hydrogen to carbon in a molecule. The empirical formula can be modified by other elements, but this is the one that counts for lab work.
It is important to remember that this doesn’t count for all hydrocarbons- there are many others with more positive values. However, when it comes down to choosing between ketones and alcohols, the preference is usually toward ketones because they are more versatile.
Know that this is not the final formula until you check it against a table of elements and compounds

There are many ways to find the Hydrocarbonwhether through a computer program, by trial and error, or by combining data from various sources.
Many websites offer them as extensions to apps for desktop and mobile devices. Video games require input from players, which comes from studying math and logic.
And finally, looking up Hydrocarbons in a book or other source can be done by combining the elements that make them up.
Check it against a table of elements and compounds
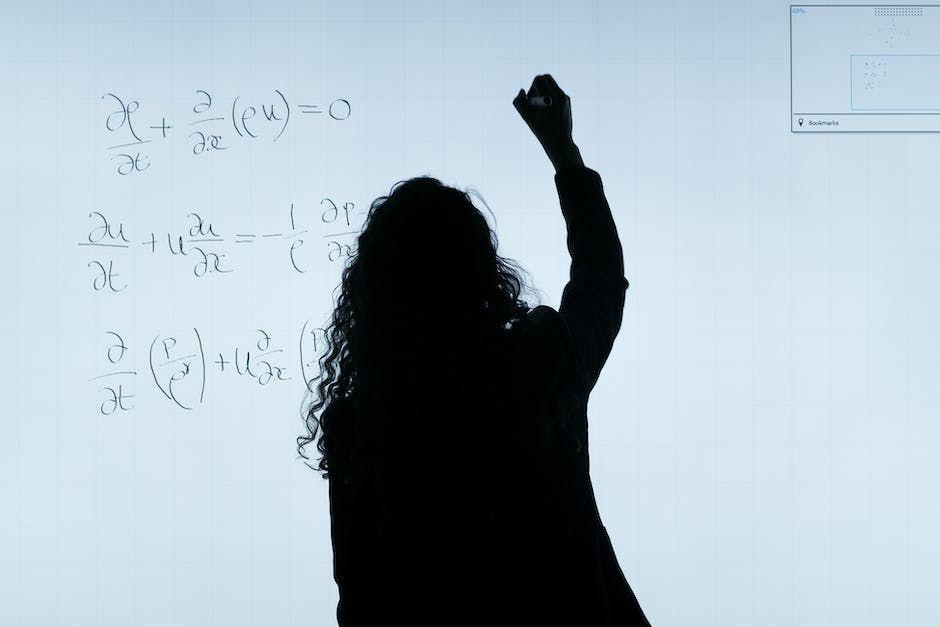
When it comes to oil and gas, there are five main elements that make up the earths hydrocarbon plethora. These elements are: rock, water, temperature, and pressure.
As you can probably imagine, these factors play a huge role in the production of oil and gas. As you also probably know, however, there are many different kinds of oil and gas. Thus, producing a formula for the toxicity of oil & gas is very difficult.
What makes the toxicity of oil & gas different from other types of fuel? There are several factors that play into the toxicity of oil & gas, such as where it is produced, what processes are used to produce it, and how it is processed.
This article will looks at these factors and give an empirical formula for the toxicity of oil & gas.

